Download the scientific poster to learn more!
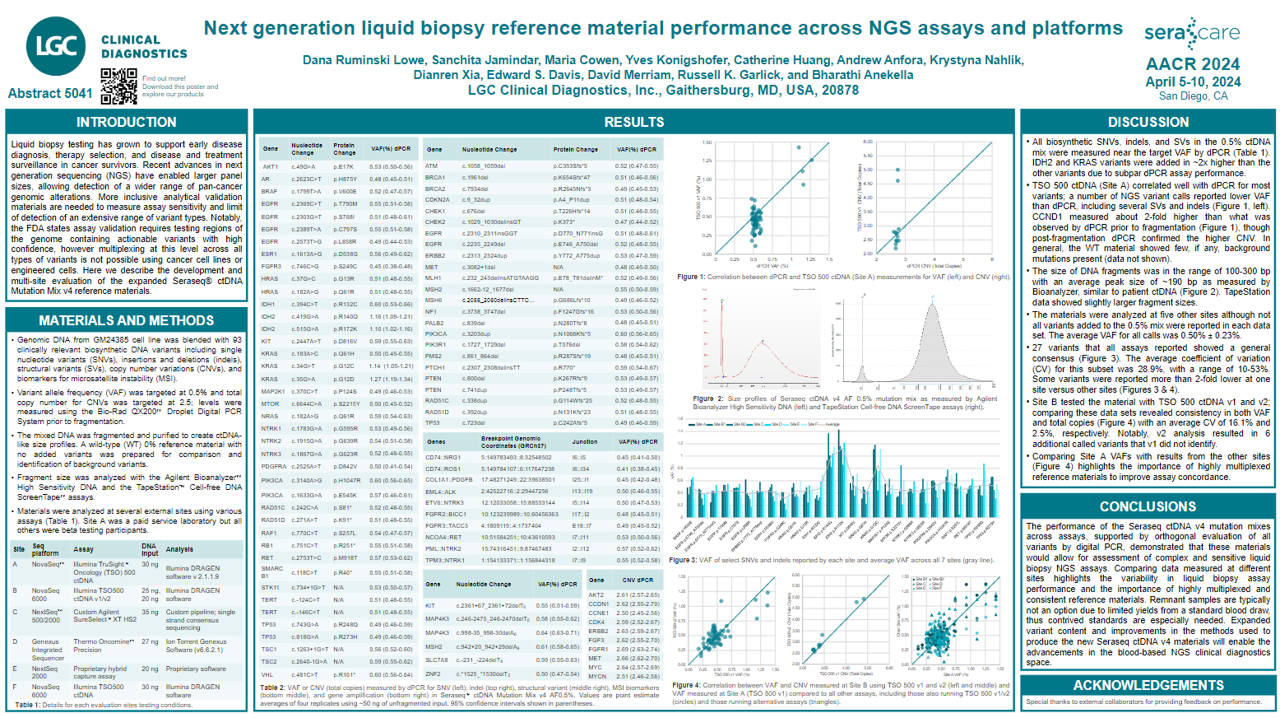
Liquid biopsy testing has grown to support early disease diagnosis, therapy selection, and disease and treatment surveillance in cancer survivors. Recent advances in next generation sequencing (NGS) have enabled larger panel sizes, allowing detection of a wider range of pan-cancer
genomic alterations. More inclusive analytical validation materials are needed to measure assay sensitivity and limit of detection of an extensive range of variant types. Notably, the FDA states assay validation requires testing regions of the genome containing actionable variants with high
confidence, however multiplexing at this level across all types of variants is not possible using cancer cell lines or engineered cells. Here we describe the development and multi-site evaluation of the expanded Seraseq® ctDNA Mutation Mix v4 reference materials.



